The FDA officially announces Remdesivir as first drug approved against Corona
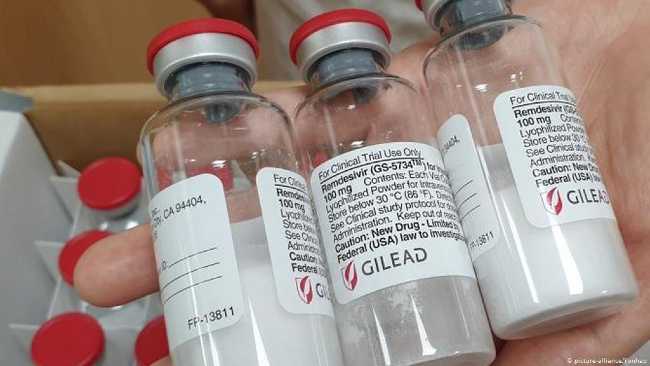
REMDESIVIR, which was cited as a “miracle drug” in the corona outbreak, has been the first drug to be given full approval by the US Food and Drug Administration for its use in the treatment of Covid-19.
As the corona virus continues to take lives around the world, vaccine and drug research continues incessantly in many countries. Hot developments continue to occur regarding the drug Remdesivir, which was developed against Ebola and suggested to be effective in the treatment of corona.
The U.S. Food and Drug Administration (FDA) has announced that Remdesivir is the first drug to be given full approval for its use in the treatment of corona virus. According to data from the US National Institute of Health, the drug which the California-based company Gilead will offer for sale under the name “VEKLURY”, reduces the recovery time from an average of 15 days to 10 days.
Full terms of use of Remdesivir, which were previously approved for use in emergencies in the spring, were also made clear. Patients aged 12 and over 40 kilograms who are being treated in hospital for the corona virus can use the drug, the FDA said.
The 5-day dose of the drug, which will be sold commercially under the name Veklury, will be 3,120 dollars (24,784 TL) in the United States. Gilead stressed that it is currently only able to produce the drug for the United States, while from the end of October it will be able to meet the purchase demands of other countries.
The FDA’s decision brought to mind a recent study by the World Health Organization on Remdesivir. After reviewing data on the treatment process of 11,266 patients from 30 countries, the who had argued that the drug in question had no effect on recovery and hospital stay in the treatment of Covid-19. (sozcu 231020)





