Historical day in Turkey: First voluntary application of covid-19 vaccine is made
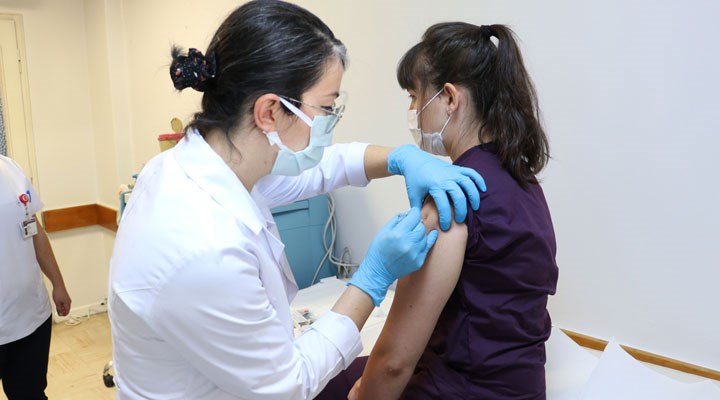
A historical stage in the corona virus vaccine, which the world is looking forward to, has been reached in Turkey. The corona virus vaccine developed by China was applied to volunteers at Hacettepe University Faculty of Medicine. The final stage has finally been reached in the corona virus vaccine, which more than 100 scientists in China have been working on.
According to a statement by the World Health Organization dated 3rd September, 8 companies in the world have started voluntary human studies of thousands of people, which is currently Phase 3, the last stage of the vaccine before production.
One of them is the inactive virus vaccine, which belongs to the Chinese company Sinovac Biotech and is popularly known as the “dead virus”. 9 countries will be included in the clinical trials of the vaccine. Minister of health Mr. KOCA had recently announced that Phase 3 trials of the vaccine would also be started in Turkey.
In countries such as Brazil and Bangladesh, where cases are very high, about 10 thousand volunteers have been vaccinated so far. The first application in Turkey was launched with three health workers at Hacettepe University Faculty of Medicine.
In the Turkish leg of Phase 3 clinical trials, approximately 1200 volunteers will be vaccinated in 25 centers in the first place, and then this number will be increased to over 10 thousand. Vaccinations and voluntary follow-up are scheduled to be completed within 2.5 months. It was stressed that this was a very historic moment. The experts say, ” We are very happy that we have contributed to something very important in the name of humanity these days when coronavirus is ravaging the world.” For Phase 3 research of this vaccine, studies were carried out for 2 months in Turkey, first at the local Ethics Committee and then at the Ethics Committee of the Ministry of Health. It is a vaccine that will be put into application after careful examination of all its data, production, side effects and all other required steps.




